WHAT IS MRI, NMRI, MRT, NMRS, NMR
MRI= Magnetic Resonance Imaging, NMRI= Nuclear Magnetic Resonance Imaging, MRT= Magnetic Resonance Tomography, NMRS= Nuclear Magnetic Resonance Spectroscopy); MRI SCANNING; CLINICAL MRI; MRI DEFINITION; MRI- CLASSIFICATION- THEORY- DEFINITION; MEANING OF MRI; MRI SCANNING TERMINOLOGY; THE MRI SCANNING; MRI SCANNING; DIFFERENCE BETWEEN NMR AND MRI; RELATION BETWEEN NMR AND MRI; MRI VS. NMR;
DIFFERENCE BETWEEN Magnetic Resonance Imaging and Magnetic Resonance Imaging, DIFFERENCE BETWEEN CT SCAN and MRI - NMR; DIFFERENCE BETWEEN X-RAY & MRI or NMR; MRI MACHINES; TYPE OF MRI MACHINES; DIFFERENT TYPE OF NMR TECHNIQUES;
Nuclear Magnetic Resonance
When the nuclear magnetic moment associated with a Nuclear Spin is placed in an external magnetic field, the different spin states are given different Magnetic Potential Energies. In the presence of the static magnetic field which produces a small amount of Spin Polarization, a radio frequency signal of the proper frequency can induce a transition between spin states. This "spin flip" places some of the spins in their higher energy state. If the radio frequency signal is then switched off, the relaxation of the spins back to the lower state produces a measurable amount of RF signal at the resonant frequency associated with the spin flip. This process is called Nuclear Magnetic Resonance (NMR).
.
A magnetic dipole moment (usually just called "Magnetic Moment") in a magnetic field will have a potential energy related to its orientation with respect to that field.
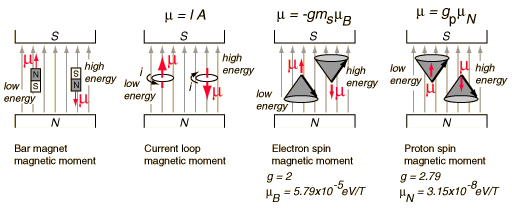
.
Note that the electron spin magnetic moment is opposite to the electron spin while the proton spin magnetic moment is in the direction of the proton spin. The electron spin or proton spin will tend to precess around the magnetic field with a frequency traditionally called the Larmor frequency. For a 1 Tesla magnetic field this Larmor frequency would be

.
.The Larmor frequency can be visualized classically in terms of the precession of the magnetic moment around the magnetic field, analogous to the precession of a spinning top around the gravity field. It can also be visualized quantum mechanically in terms of the quantum energy of transition between the two possible spin states for spin 1/2. This can be expressed as a photon energy according to the Planck relationship. The magnetic potential energy difference is hn = 2mB. The short table of Larmor frequencies below is from Hobbie, Ch 17 and Becker. An extensive list including the magnetic moments and Larmor frequencies of most elements can be found in Appendix A of Becker.
| Particle | Spin | s-1T-1 | |
| Electron | 1.7608 x 1011 | ||
| Proton | 42.5781 MHz/T | ||
| Deuteron | |||
| Neutron | 29.1667 MHz/T | ||
| 11.2618 MHz/T | |||
| 17.2349 MHz/T | |||
.
The Larmor frequency of the electron spin is in the microwave region of the electromagnetic spectrum and is used in electron spin resonance.
The Larmor frequency of the electron spin is in the microwave region of the electromagnetic spectrum and is used in electron spin resonance.
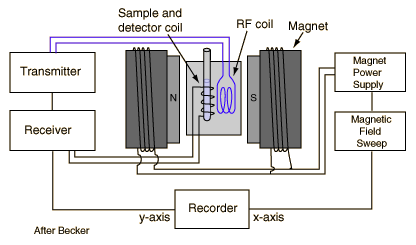
The precession of the proton spin in the magnetic field is the interaction which is used in proton NMR. As a practical technique, a sample containing protons (hydrogen nuclei) is placed in a strong magnetic field to produce partial polarization of the protons. A strong RF field is also imposed on the sample to excite some of the nuclear spins into their higher energy state. When this strong RF signal is switched off, the spins tend to return to their lower state, producing a small amount of radiation at the Larmor frequency associated with that field. The emission of radiation is associated with the "spin relaxation" of the protons from their excited state. It induces a radio frequency signal in a detector coil which is amplified to display the NMR signal.
.
.
Since the Larmor frequency of the detected signal is proportional to the applied magnetic field, changing the magnitude of that field produces a different detected frequency. Placing a magnetic field gradient across a sample allows you to locate the source of the proton NMR signal in the sample. This is used to great advantage in the medical imaging process known as Magnetic Resonance Imaging.
Nuclear SpinIt is common practice to represent the total angular momentum of a nucleus by the symbol I and to call it "nuclear spin". For electrons in atoms we make a clear distinction betweenelectron spin and electron orbital angular momentum, and then combine them to give thetotal angular momentum. But nuclei often act as if they are a single entity with intrinsic angular momentum I. Associated with each nuclear spin is a Nuclear Magnetic Moment which produces magnetic interactions with its environment. . The nuclear spins for individual protons and neutrons parallels the treatment of electron spin, with spin 1/2 and an associated magnetic moment. The magnetic moment is much smaller than that of the electron. For the combination neutrons and protons into nuclei, the situation is more complicated. A characteristic of the collection of protons and neutrons (which are fermions) is that a nucleus of odd mass number A will have a half-integer spin and a nucleus of even A will have integer spin. The suggestion that the angular momenta of nucleons tend to form pairs is supported by the fact that all nuclei with even Z and even N have nuclear spin I=0. For example, in the nuclear data table for iron below, all the even A nuclides have spin I=0 since there are even numbers of both neutrons and protons. The half-integer spins of the odd-A nuclides suggests that this is the nuclear spin contributed by the odd neutron. Isotopes of Iron
The nuclear data of cobalt, just above iron in the periodic table, shows dramatically different nuclear spins I. The nuclides with even neutron number show half-integer spins associated with the odd proton, while those with odd neutron number show large integer spins associated with the two nucleons which are unpaired.
| Index Nuclear Structure Concepts Reference Krane Ch 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Nuclear Magnetic MomentsAssociated with each nuclear spin is a magnetic moment which is associated with the angular momentum of the nucleus. It is common practice to express these magnetic moments in terms of the nuclear spin in a manner parallel to the treatment of the magnetic moments of electron spin and electron orbital angular momentum. For the electron spin and orbital cases, the magnetic moments are expressed in terms of a unit called a Bohr magneton which arises naturally in the treatment of quantized angular momentum. 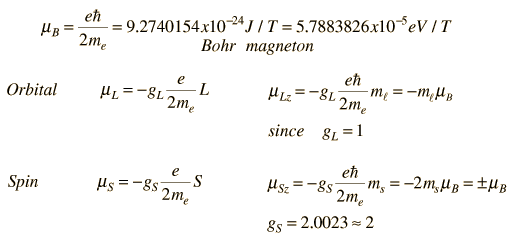 For the nuclear case we proceed in a parallel manner. The nuclear magnetic moment is expressed in terms of the nuclear spin in the form 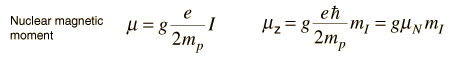 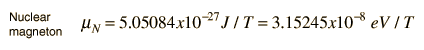 For free protons and neutrons with spin I =1/2, the magnetic moments are of the form 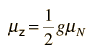 Neutron: g = -3.8260837 +/- 0.0000018 The proton g-factor is far from the gS = 2 for the electron, and even the uncharged neutron has a sizable magnetic moment! For the neutron, this suggests that there is internal structure involving the movement of charged particles, even though the net charge of the neutron is zero. If g=2 were an expected value for the proton and g=0 were expected for the neutron, then it was noted by early researchers that the the proton g-factor is 3.6 units above its expected value and the neutron value is 3.8 units below its expected value. This approximate symmetry was used in trial models of the magnetic moment, and in retrospect is taken as an indication of the internal structure of quarks in the standard model of the proton and neutron. Note that the maximum effective magnetic moment of a nucleus in nuclear magnetons will be the g-factor multiplied by the nuclear spin. For a proton with g = 5.5857 the quoted magnetic moment is m = 2.7928 nuclear magnetons.
Data from V. S. Shirley, Table of Isotopes, Wiley, New York, 1978, Appendix VII.
|
What is The Chemical Applications of NMR, Nuclear Magnetic Resonance;
Chemical Applications of NMR
The versatility of Nuclear Magnetic Resonance (NMR) spectroscopy has made it a widespread tool in chemistry for the study of chemical structure. In additional to the one-dimensional NMR spectroscopy used to study chemical bonds, two dimensional approaches have been developed for the determination of the structure of complex molecules like proteins. Time domain NMR spectroscopy is used to study molecular dynamics in solutions. NMR of solid samples can help determine molecular structures. There are NMR methods for measuring diffusion coefficients.
NMR spectroscopy has contributed enormously to chemical knowledge. A wide range of techniques has been used with a range of magnetic fields including high-field superconducting magnets. NMR frequencies from 60 to 800 MHz have been used for hydrogens, compared to the range of about 15 to 80 MHz for medical Magnetic Resonance Imaging (MRI).
One of the major sources of chemical information is the measurement of Chemical Shifts in high-resolution NMR spectroscopy. The chemical shifts are a very sensitive probe of the chemical environment of the resonating nuclei.



No comments:
Post a Comment